
Essential Experiments for Chemistry PDF: A Comprehensive Guide
This guide offers a curated collection of essential chemistry experiments, conveniently compiled in PDF format. It serves as a valuable resource for students and educators, providing clear instructions, safety guidelines, and insightful explanations of core chemical principles. Explore time-tested experiments designed to strengthen understanding and spark curiosity.
Chemistry, at its heart, is an experimental science. The theoretical concepts that underpin our understanding of the world are derived from, and continuously validated by, meticulous observation and experimentation. This section introduces the fundamental role that experiments play in the study of chemistry. It emphasizes the importance of hands-on experience in solidifying theoretical knowledge and developing critical thinking skills. From simple reactions to complex analyses, experiments provide students with a tangible connection to the abstract principles they learn in lectures.
This introduction also highlights the broader educational benefits of engaging in chemistry experiments. Beyond simply illustrating chemical concepts, these experiments foster skills in problem-solving, data analysis, and scientific communication. Through careful planning, execution, and interpretation of results, students learn to think like scientists, developing the ability to formulate hypotheses, design experiments to test those hypotheses, and draw meaningful conclusions from their observations. Moreover, the collaborative nature of many laboratory activities promotes teamwork and communication skills, essential for success in both academic and professional settings. This section sets the stage for a journey into the world of practical chemistry, where curiosity and careful observation lead to deeper understanding.

Safety Protocols in Chemistry Labs
The chemistry laboratory is a dynamic environment where exciting discoveries can be made, but it’s also a place where safety must be paramount. Understanding and adhering to strict safety protocols are crucial for protecting oneself and others from potential hazards. This section outlines the essential safety guidelines that must be followed in any chemistry lab, regardless of the experiment being performed.
These protocols encompass a range of measures, from wearing appropriate personal protective equipment (PPE) such as safety goggles and lab coats, to handling chemicals with care and disposing of waste properly; It emphasizes the importance of knowing the location and proper use of safety equipment like fire extinguishers, eyewash stations, and safety showers. Furthermore, this section underscores the need for thorough preparation before beginning any experiment, including understanding the potential hazards associated with the chemicals being used and the procedures being followed. By fostering a culture of safety consciousness, we can ensure that the chemistry lab remains a safe and productive learning environment. Always review the safety data sheets (SDS) for each chemical before use.
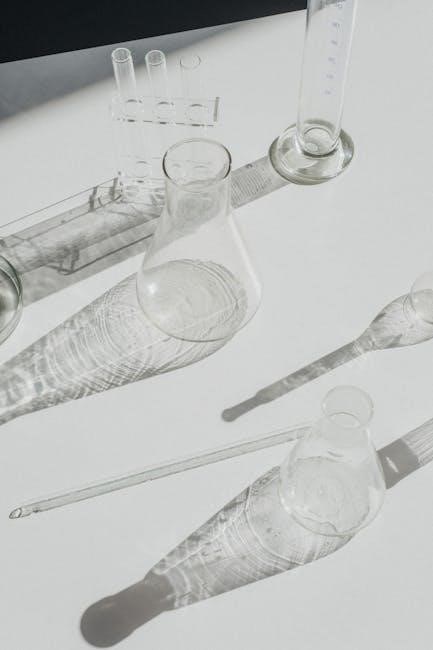
Identifying Basic Chemistry Equipment and Glassware

A solid foundation in chemistry begins with familiarity with the tools of the trade. This section focuses on identifying and understanding the function of basic chemistry equipment and glassware commonly found in a laboratory setting. Mastering the names and uses of these items is essential for conducting experiments accurately and safely.
From beakers and Erlenmeyer flasks to test tubes and graduated cylinders, each piece of glassware serves a specific purpose in measuring, mixing, and reacting chemicals. We will explore the proper techniques for handling glassware to prevent breakage and contamination. In addition to glassware, we will cover essential equipment such as Bunsen burners, hot plates, balances, and pipettes, explaining their roles in heating, measuring, and transferring substances. Knowing the capabilities and limitations of each tool will enable you to perform experiments with confidence and obtain reliable results. Furthermore, proper cleaning and maintenance procedures for each item will be discussed to ensure their longevity and accuracy.
Fundamental Laboratory Techniques
Proficiency in fundamental laboratory techniques is crucial for success in chemistry; This section delves into the essential skills needed to conduct experiments effectively and safely. These techniques form the backbone of all experimental work, ensuring accurate data collection and reliable results.
We will cover techniques such as accurate weighing of chemicals using balances, proper measurement of liquids using graduated cylinders and pipettes, and effective mixing and stirring methods. Filtration techniques for separating solids from liquids will also be explored, along with distillation for purifying liquids. Mastering titration, a key technique for determining the concentration of a solution, will be emphasized. Furthermore, proper heating and cooling methods will be discussed, including the use of Bunsen burners, hot plates, and ice baths. By mastering these fundamental techniques, students will gain the confidence and skills necessary to conduct a wide range of chemistry experiments with precision and accuracy. Emphasis will be placed on safety procedures for each technique to ensure a safe and productive laboratory environment.

Accuracy and Precision in Experimental Data Evaluation

Evaluating experimental data is a critical step in the scientific process. Understanding the concepts of accuracy and precision is fundamental to interpreting results and drawing meaningful conclusions. Accuracy refers to how close a measurement is to the true or accepted value, while precision describes the reproducibility of a series of measurements.
This section will guide you through the methods of assessing accuracy and precision, including calculating percent error and standard deviation. We will explore the sources of error in experimental measurements, such as systematic and random errors, and discuss strategies for minimizing these errors. Statistical analysis techniques, such as calculating averages and confidence intervals, will be introduced to help you determine the reliability of your data. Proper data recording and presentation methods will also be emphasized, including the use of significant figures and appropriate units. By mastering these skills, you will be able to critically evaluate your experimental data, identify potential sources of error, and draw valid conclusions based on your findings. This section will also cover how to use software to evaluate data.
Core Chemical Principles Illustrated Through Experiments
This section delves into fundamental chemical principles, demonstrating how they are brought to life through carefully designed experiments. We will explore core concepts like stoichiometry, chemical kinetics, thermodynamics, and equilibrium. Each principle will be illustrated with specific experiments that allow for hands-on learning and a deeper understanding of the underlying theory.
For example, experiments involving titration will showcase stoichiometry in action, allowing you to quantify the relationships between reactants and products. Chemical kinetics will be explored through experiments that measure reaction rates and investigate factors affecting those rates, such as temperature and concentration. Thermodynamic principles will be demonstrated through experiments involving calorimetry, where you can measure heat transfer and determine enthalpy changes. Equilibrium will be illustrated through experiments that explore Le Chatelier’s principle and its impact on chemical reactions. Through these experiments, you’ll connect theoretical concepts with real-world observations, solidifying your understanding of core chemical principles and enhancing your problem-solving abilities. The experiments will allow you to grasp the principles and understand the practical applications. You will also understand the laws of chemistry.

Examples of Engaging Chemistry Practicals
This section highlights several engaging and captivating chemistry practicals designed to ignite curiosity and foster a deeper appreciation for the subject. Experiments like the “Cartesian diver” demonstrate principles of buoyancy and pressure in an exciting way. The creation of “disappearing ink” introduces reversible reactions and the concept of chemical equilibrium. Another captivating practical involves synthesizing polymers, showcasing the building blocks of plastics and their unique properties.

Exploring acid-base reactions through colorful titrations provides a visual and interactive understanding of neutralization. Investigating the properties of different metals through flame tests reveals the characteristic emission spectra of elements. Building a voltaic cell allows students to witness the conversion of chemical energy into electrical energy, demonstrating redox reactions in action. These engaging practicals not only reinforce theoretical concepts but also encourage critical thinking, problem-solving, and teamwork. Each practical is designed to be hands-on, allowing students to actively participate in the learning process and develop a lasting interest in chemistry. These hands-on approaches will help you gain a better understanding of chemical reactions and the states of matter.
The Role of Water as a Solvent in Chemical Experiments
Water plays a pivotal role in a vast array of chemical experiments, primarily serving as a solvent. Its unique properties, such as its polarity and ability to form hydrogen bonds, make it an exceptional medium for dissolving a wide range of substances. Water’s capacity to dissolve ionic compounds stems from its ability to surround ions with its polar molecules, effectively separating them and preventing them from re-associating. This property is crucial for facilitating reactions between ionic species in solution.
Moreover, water’s ability to dissolve polar covalent compounds arises from similar interactions, where the slightly charged ends of water molecules interact favorably with the polar regions of the solute molecules. The use of water as a solvent simplifies the handling and mixing of chemicals, allowing for controlled reactions. It also facilitates the movement of reactants and products, enhancing reaction rates. The high heat capacity of water helps to moderate temperature changes during exothermic or endothermic reactions, contributing to more stable and predictable experimental conditions. Without water’s solvent capabilities, many fundamental chemical experiments would be far more challenging, if not impossible, to conduct effectively. The use of water is essential for many chemical experiments.
Digital Laboratories in Modern Chemistry Education
Digital laboratories are revolutionizing modern chemistry education by offering interactive and accessible learning experiences. These virtual environments simulate real-world laboratory settings, allowing students to conduct experiments without the constraints of physical equipment or safety concerns. Digital labs provide a safe space to explore chemical reactions, manipulate variables, and observe outcomes in a controlled manner. They often include interactive simulations, 3D visualizations, and data analysis tools, enhancing comprehension and engagement.
One of the key benefits of digital laboratories is their ability to provide immediate feedback and guidance. Students can instantly assess their understanding, identify errors, and refine their experimental techniques. Furthermore, digital labs can replicate experiments that are too dangerous or costly to perform in a traditional classroom. They also offer flexibility, allowing students to access and complete experiments at their own pace and from any location. This accessibility is particularly valuable for remote learning and students with disabilities.
As technology continues to advance, digital laboratories will likely play an increasingly important role in chemistry education, preparing students for the challenges and opportunities of the 21st century.

